Braille
Creation of Braille for pharmaceutical packaging materials
- Implementation of braille texts
- Compliance with the manufacturer’s technical specifications
- Internal quality control
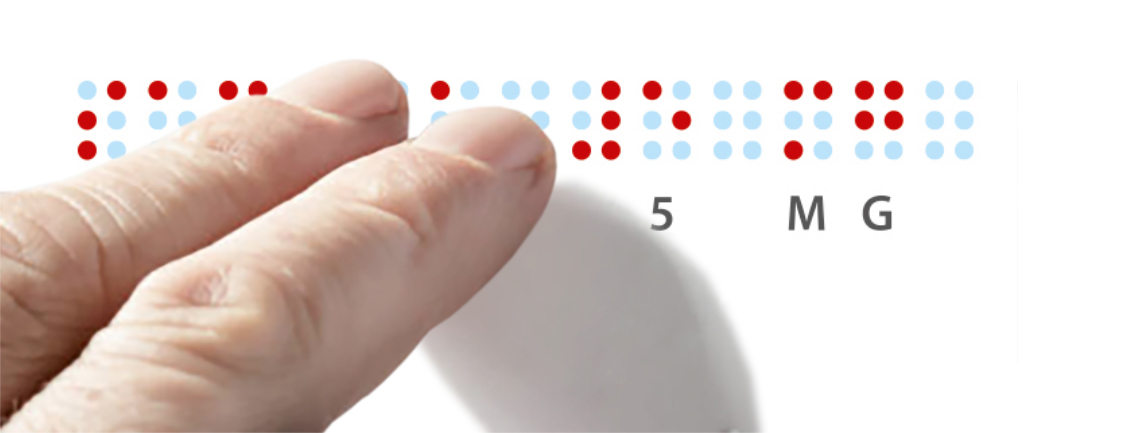
Braille is required by law on many packages. We implement the required Braille according to the manufacturer’s specifications and the Pharma Braille requirements for the labelling of drugs and medical devices in your pharmaceutical packaging design.
In doing so, we ensure the technical feasibility and readability of the printed information. We are happy to translate plain text into Braille – in European and non-European languages.
The implementation of brochures or more extensive texts in Braille is also part of our scope of services.
